Anas Abuzoor; Abir T. El-Alfy, PhD, MS; Kristin Busse, PharmD, BCPS
WMJ. 2022;122:P1. Published online January 30, 2023.
Auvelity was approved for the treatment of major depressive disorder (MDD) in adults on August 19, 2022, by the US Food and Drug Administration.
AUVELITY MECHANISM
Dextromethorphan (DXM) acts as an uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, sigma 1 receptor agonist, and serotonin reuptake inhibitor, while bupropion (BUP) acts as a norepinephrine-dopamine reuptake inhibitor and nicotinic receptor antagonist. BUP is also a potent inhibitor of cytochrome P450 2D6 (CYP2D6), and thereby inhibits the metabolism of dextromethorphan to dextrorphan, leading to enhanced bioavailability of DXM. The exact mechanism of action of DXM in the treatment of depression is unknown; however, it is thought to act through modulation of glutamate signaling. When administered together as DXM/BUP, the elimination half-life of DXM is 22 hours and the elimination half-life of BUP is 15 hours.
DOSAGE AND ADMINISTRATION
DXM/BUP is available in the form of extended-release oral tablets containing a fixed dose combination of 45 mg DXM hydrobromide and 105 mg BUP hydrochloride per tablet, given once daily for 3 days, then increased to twice daily thereafter, administered at least 8 hours apart. Note that the typical dose of BUP for MDD is 300-450 mg/day. Patients who are CYP2D6 poor metabolizers should be given a once-daily dose. Cost: $561/30 tablets.
SIDE EFFECTS
Dizziness, headache, diarrhea, somnolence, dry mouth, sexual dysfunction, hyperhidrosis, anxiety, constipation, and insomnia.
WARNING SIGNS AND INTERACTIONS
Auvelity comes with a boxed warning for increased risk of suicidal thoughts and behaviors in pediatric and young adult patients. It is contraindicated in patients with a seizure or eating disorder. Drug-drug interactions may exist with other CYP2D6 substrates, inhibitors, and inducers.
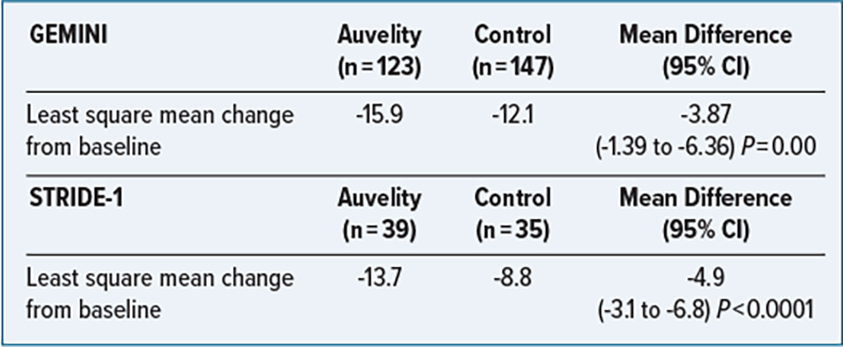
CLINICAL TRIALS
GEMINI: Randomized control trial, double-blind, placebo-controlled.
Population: Adults age 18 to 65 years; must meet the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for MDDl, have a Montgomery-Asberg Depression Rating Scale (MADRS) score of ≥ 25, a Clinical Global Impressions scale severity of illness (CGI-S) score ≥ 4, and a body masss index between 18 and 40. Patients with suicide risk, history of treatment resistance in current depressive episode or nonpharmacologic intervention (electroconvulsive therapy, vagus nerve stimulation, transcranial magnetic stimulation) in the past 6 months, and those who were pregnant were excluded.
STRIDE-1: Randomized control trial, double-blind, parallel-group.
Population: Adults age 18-65 who have a confirmed diagnosis of MDD, a current moderate or greater severity episode using MADRS, and CGI-S ≥ 4. Patients with a history of any of the following were excluded: bipolar disorder, panic disorder, obsessive-compulsive disorder, treatment-resistant depression (2 failed antidepressant treatments), substance use disorder, psychotic disorder, risk of suicide, seizure disorder.
Primary Endpoint: Change in MADRS total score from baseline measured as least square mean change to week 6.
CONCLUSIONS
Auvelity has demonstrated efficacy in management of moderate to severe major depressive disorder when compared to placebo and BUP, respectively. Auvelity was generally well-tolerated in comparison with BUP.