Brandon Johnson; Kristin Busse, PharmD, BCPS; Abir T. El-Alfy, PhD, MS
WMJ. 2025;124:P1. Published online March 10, 2025.
Download full-text pdf.
On September 26, 2024, the United States Food and Drug Administration (FDA) approved a new drug application, submitted by Bristol-Myers Squibb, for the combination product xanomeline and trospium chloride (Cobenfy) – the first antipsychotic drug approved to treat schizophrenia by targeting the cholinergic receptors rather than the dopamine/serotonin receptors. Cobenfy is the first drug to take a new approach in the treatment of schizophrenia and offers an alternative to previously approved antipsychotics.
COBENFY MECHANISM OF ACTION
Cobenfy consists of two active pharmaceutical ingredients: xanomeline and trospium chloride. Xanomeline acts as a muscarinic agonist for receptors M1 through M5, all at similar affinity. However, xanomeline exhibits a higher agonist activity at the M1 and M4 receptors, specifically. In contrast, trospium chloride is a muscarinic antagonist that primarily acts in the peripheral tissues.
Together, the combination of trospium and xanomeline leads to selective agonism at the muscarinic receptors M1 and M4. Furthermore, the addition of trospium leads to less adverse effects of xanomeline muscarinic agonism in the peripheral tissues. The mechanism of action of Cobenfy in the treatment of schizophrenia is not entirely clear, but it is thought to be related to the agonist activity at M1 and M4 receptors in the central nervous system.
FDA APPROVED INDICATION: SCHIZOPHRENIA
Approval of Cobenfy (xanomeline-trospium) was granted by the FDA on September 26, 2024, based on the results of the EMERGENT-2 and EMERGENT-3 phase III trials.
CLINICAL TRIAL DATA
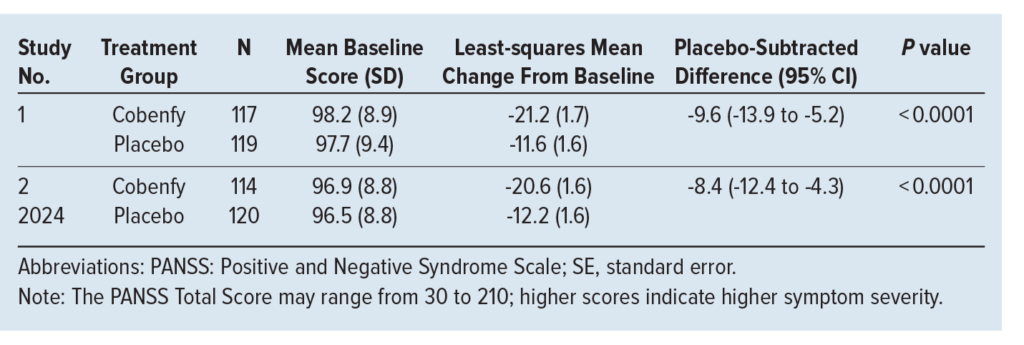
EMERGENT-2 Trial (Study 1, 2023) and EMERGENT-3 Trial (Study 2, 2024)
Both studies were phase 3 randomized, double-blind, placebo-controlled trials with identical designs conducted in adult patients with a diagnosis of schizophrenia according to the DSM-5 criteria. Patients (N = 470, combined for both studies) received Cobenfy or placebo for 5 weeks. The Cobenfy group initially was started on a dose of 50 mg/20 mg by mouth twice daily for 2 days, then, if tolerated, increased to 100 mg/20 mg by mouth twice daily for the rest of week 1 (days 3-7). On day 8, the dose was increased to 125 mg/30 mg by mouth twice daily for the remainder of the study period, unless unable to be tolerated. Demographics and baseline characteristics were similar between both groups in both studies.
The primary outcome was the change from baseline in the Positive and Negative Syndrome Scale (PANSS) total score. The PANSS measures various symptoms of schizophrenia and is rated by a clinician on a 7-point scale, with 1 indicating absence of symptoms and 7 indicating extreme symptoms. As seen in the Table, Cobenfy demonstrated a significant reduction in the PANSS total score in both studies. Subgroup analysis suggested no difference in response based on race, sex, or age.
DOSING AND ADMINISTRATION
Recommended Dosing
- Initial: 50/20 mg by mouth twice daily for at least 2 days, then increase to 100/20 mg by mouth twice daily
- Maximum dosage: 125/30 mg by mouth twice daily
- Geriatric maximum dosage: 100/20 mg by mouth twice daily
Administration
- Do not open the capsules
- Administer at least 1 hour before meals or at least 2 hours after meals
Cost
- Average wholesale price: $37 per capsule
- Manufacturer annual cost: $22,500 per year
ADVERSE EFFECTS
Trial results reported many possible adverse effects that may play a role in the decision to prescribe and utilize this newer agent. The following adverse effects have demonstrated high incidence (>10%) or statistically significant differences between Cobenfy and placebo:
- Nausea
- Dyspepsia
- Constipation
- Vomiting
- Hypertension
CONTRAINDICATIONS
- Urinary retention
- Child-Pugh Class B or Class C hepatic impairment
- Gastric retention
- Untreated narrow-angle glaucoma
- Any history of hypersensitivity to Cobenfy or any of its ingredients
DRUG/FOOD INTERACTIONS
Cobenfy is metabolized by CYP2D6 and eliminated by active tubular secretion. In addition, Cobenfy inhibits CYP3A4 and P-Glycoprotein.
- Use of CYP2D6 inhibitors may increase the serum concentration of xanomeline. Examples of CYP2D6 inhibitors include fluoxetine, paroxetine, cannabidiol, and bupropion.
- Use of CYP3A4 substrates may increase their serum concentrations. Examples of CYP3A4 substrates include cyclosporine, tacrolimus, macrolide antibiotics, and certain antidepressants.
CONCLUSIONS
The approval of Cobenfy is a significant development in the treatment of schizophrenia and provides the first new mechanism of action for treating schizophrenia in decades. Participants in the EMERGENT trials experienced acute psychosis and showed a significant reduction in PANSS score. Additionally, Cobenfy has a different side effect profile from existing antipsychotics. Due to its novel mechanism of action, the existing antipsychotic side effects, such as weight gain, sedation, metabolic syndrome, and extrapyramidal symptoms, are avoided.
Ultimately, Cobenfy offers an alternative agent for patients who may not respond to existing treatment or for those who cannot tolerate the side effect profile of existing agents. From an accessibility standpoint, the manufacturer has given a list price of $22,500 for a year’s supply, though it remains to be seen how much insurances will cover for patients.