Kristin Busse, PharmD, BCPS; Abir T. El-Alfy, PhD, MS
WMJ. 2022;121:P3. Published online November 10, 2022.
Paxlovid was authorized for emergency use on December 22, 2021, by the US Food and Drug Administration (FDA) for treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progression to severe COVD-19, including hospitalization or death. Since it is not FDA-approved at this time, Paxlovid is considered an investigational drug.
PAXLOVID MECHANISM
Paxlovid is a combination of nirmatrelvir, a SARS-CoV-2 main protease inhibitor, and ritonavir, an HIV-1 protease inhibitor and CYP3A inhibitor. Nirmatrelvir stops the virus from replicating. Ritonavir helps to keep nirmatrelvir at higher concentrations in the body for a longer time period by inhibiting its metabolism.
DOSAGE AND ADMINISTRATION
- Nirmatrelvir must be co-administered with ritonavir.
- Initiate treatment as soon as possible after diagnosis and within 5 days of symptom onset.
- Administer 300 mg nirmatrelvir (2 x 150 mg tablets) with 100 mg ritonavir (1 tablet): all three taken together twice daily for 5 days, with or without food.
- Dose reduce to 150 mg nirmatrelvir with 100 mg ritonavir twice daily for moderate renal impairment (eGFR ≥ 30 to < 60 mL/min).
CLINICAL TRIALS
The primary data supporting the emergency use authorization for Paxlovid are from EPIC-HR, a randomized, double-blind, placebo-controlled trial studying Paxlovid for the treatment of nonhospitalized symptomatic adults with a laboratory confirmed diagnosis of SARS-CoV-2 infection.
Population: Adults 18+ years with a pre-specified risk factor for progression to severe disease OR 60+ years regardless of prespecified risk factor. No patients had previously been infected with COVID-19 or had received a COVID-19 vaccine.
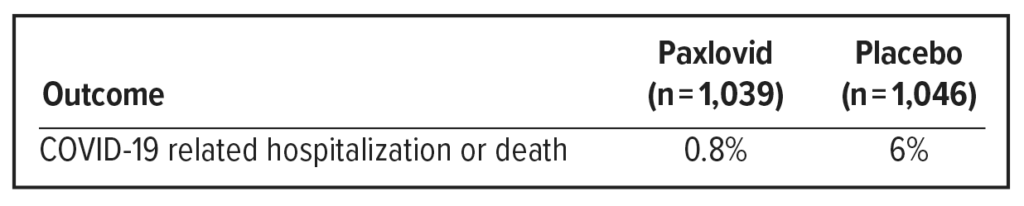
Paxlovid significantly reduced the proportion of people with COVID-19 related hospitalization or death from any cause by 88% compared to placebo.
DRUG INTERACTIONS
- Paxlovid is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious and/or life-threatening reactions.
- Paxlovid is contraindicated with drugs that are potent CYP3A inducers where significantly reduced nirmatrelvir or ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance.
- The importance of these interactions should not be taken lightly, given that CYP3A4 enzymes are responsible for metabolism of > 50% of medications on the market. For example, tacrolimus, a potent immunosuppressant, must be held during Paxlovid treatment.
- The Centers for Disease Control and Prevention (CDC) has developed an eligibility checklist, including guidance on drug interactions, that some clinicians may find helpful when determining whether to prescribe Paxlovid.
- The University of Liverpool COVID-19 Drug Interaction Checker is a free online tool that can be used as an additional resource.
AVAILABILITY
In an effort to make treatment more accessible, Department of Health and Human Services (DHHS) launched a website for COVID-19 Test to Treat locations. Patients with COVID-19 symptoms can be tested, receive a prescription for Paxlovid, and have it filled all in one location. Additionally, pharmacists have been authorized to prescribe Paxlovid if they have access to recent labs (within 12 months) and an updated medication list.
CONCLUSION
Paxlovid is available under an emergency use authorization to treat mild-to-moderate COVID-19 in patients at risk for hospitalization or death. It has not been FDA approved yet but is available at many pharmacies. Use of the eligibility checklist and the drug interaction tool may be of use to some clinicians.