Kong Choua Thao; Abir T. El-Alfy, PhD, MS; Kristin Busse, PharmD, BCPS
WMJ. 2023;122:P2. Published online August 28, 2023.
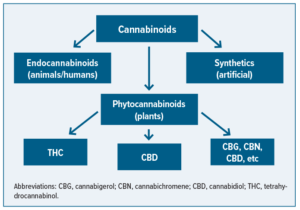
The implementation of the 2018 Agriculture Improvement Act (2018 Farm Bill) legalized the commercial production of hemp. Prior to this, the Controlled Substance Act categorized hemp as a schedule I drug. Hemp is from the same species of plant, Cannabis sativa, as marijuana, but the key difference lies in the content of the psychoactive component delta-9 tetrahydrocannabinol (THC). Hemp is defined as a cannabis plant having very low levels of THC, <0.3%, meaning it does not have any psychoactive properties and detectability in urine screenings. Cannabidiol (CBD), a phytocannabinoid derived mainly from hemp, had captured the attention of researchers and consumer market through CBD legalization. There has been a surge in the number of clinical trials assessing the therapeutic effects of CBD and the accessibility of CBD to the public for a variety of health conditions. However, there is a lack of clinical evidence that supports the use of CBD. This update provides a high-level overview of different types of cannabidiol products and how they are regulated.
EPIDIOLEX
Epidiolex is the only form of CBD approved by the US Food and Drug Administration (FDA) available through a prescription to treat seizures.
Indications: FDA approved on June 25, 2018 for the treatment of seizures associated with Lennox-Gastaut syndrome (LGS), Dravet syndrome (DS), or tuberous sclerosis complex (TSC) in patients 1 years of age and older.
Mechanism of Action: Precise mechanisms by which Epidiolex exerts its anticonvulsant effect in humans are unknown and appears to not be related with cannabinoid receptors.
Adverse Reactions: Transaminase elevations, decrease appetite, somnolence, diarrhea, poor quality sleep, and infections.
Drug Interactions: Strong inducer of CYP3A4 or CYP2C19. When combined with everolimus, reduce starting dose of everolimus.
TYPES OF CANNIBIDIOL PRODUCTS
- Full-Spectrum: contains all parts of the cannabis plant, <0.3% THC content
- Broad-Spectrum: contains most of the cannabis plant, trace amounts of THC
- Isolate: contains pure form of CBD and no other cannabis plant compound
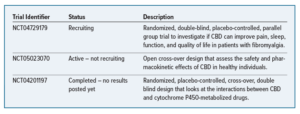
CLINICAL TRIALS OF CANNABIDIOL PRODUCTS
On December 2, 2022, Medical Marijuana and Cannabidiol Research Expansion Act was passed to encourage valid scientific and clinical research on marijuana and CBD. The purpose of this is to help promote commercial production of FDA-approved drugs derived from CBD. Below are some current studies looking at potential indications, safety, and drug interaction risk of CBD.
FDA RESPONSE TO THREE CITIZEN PETITIONS
On January 26, 2023, the FDA denied the requests in three citizen petitions, from the Consumer Healthcare Products Association, the Council for Responsible Nutrition, and the Natural Products Association, to market CBD as dietary supplements. The FDA responded that there is insufficient scientific evidence that meets dietary supplements safety standards. In addition, the FDA plans to work with Congress to develop new regulatory pathway for CBD.
CONCLUSION
With the emergence of legal cannabinoids in a variety of preparations available direct to consumers, healthcare providers are encouraged to discuss usage of these products with their patients. At this point, little data are available to support the effective use of these products. The FDA does not plan to allow CBD products to be marketed as dietary supplements. Until future studies are completed and new regulations are implemented, continued discussions with patients on the drug interactions, possible positive urine drug tests (with CBD products >0.03% THC), and adverse reactions are key.