Kristin Busse, PharmD, BCPS; Abir T. El-Alfy, PhD, MS
WMJ. 2022;121:P2. Published online June 6, 2022.
The past few years have witnessed several clinical studies that investigated the potential benefits of fluvoxamine maleate in treating COVID-19 patients. In December 2021, David R. Boulware, MD, MPH, a physician and researcher at the University of Minnesota, requested an emergency use authorization (EUA) for the use of fluvoxamine for early treatment of COVID-19 outpatients to prevent their clinical deterioration.
INDICATIONS & MECHANISM
Fluvoxamine is an antidepressant drug that acts as a selective serotonin reuptake inhibitor (SSRI). It has been used in the United States for the treatment of depression, obsessive compulsive disorder, anxiety, and panic attacks.
FLUVOXAMINE USE IN COVID-19 PATIENTS
The use of fluvoxamine maleate in COVID-19 patients originates from the drug repurposing approach. Such approach is adopted to promptly discover therapeutic drugs for COVID-19 patients from existing inexpensive ones. Increased data suggested that the use of antidepressants—especially SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRI)—might reduce the clinical deterioration of COVID-19 patients. Fluvoxamine emerged on top of the list because of several small clinical trials that reported its potential benefit to prevent deterioration in early stage COVID-19 outpatients.
POTENTIAL MECHANISMS FOR COVID-19
The protective effect of fluvoxamine against severe COVID-19 outcomes may be mediated through antiviral, anti-inflammatory, and immune-modulating mechanisms.
Antiviral mechanisms:
- Agonist action on sigma 1 receptors. These ER cellular factors play a role in inhibiting viral replication during early stage of the infection as well as contribute to the anti-inflammatory effect of fluvoxamine
- Interference with viral trafficking and regulation of inositol signaling pathways
Anti-inflammatory and immune modulation mechanisms:
- Platelet aggregation, mast cell degranulation inhibition
- Increasing anti-inflammatory cytokines and reducing expression of inflammatory ones
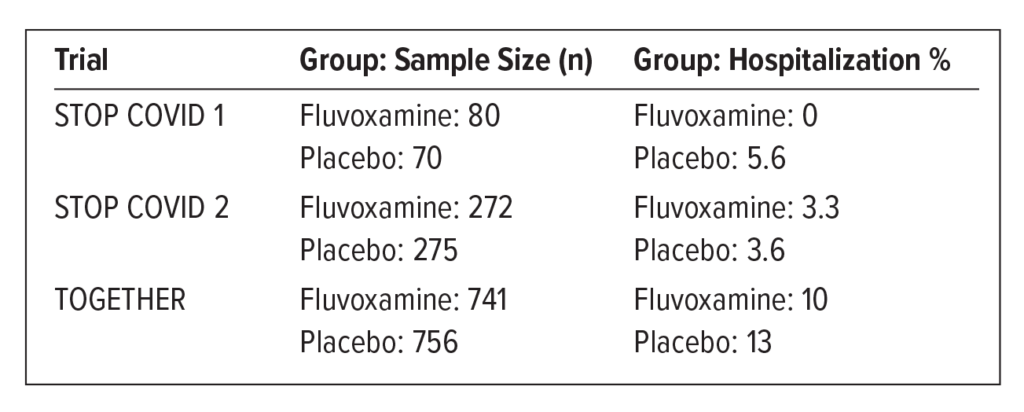
CLINICAL TRIALS
Several clinical trials examined the utility of fluvoxamine in COVID-19 outpatients. The STOP COVID trials measured clinical deterioration. STOP COVID 1 had several limitations, including small sample size and lack of randomization. STOP COVID 2 was “terminated early for futility.” The EUA submission was based on the results of the TOGETHER trial.
TOGETHER Trial: Multicenter, placebo-controlled, randomized. Endpoint measured: requiring more than 6 hours of observation or transferring to a hospital within 28 days because of COVID.
FDA DENIES EUA REQUEST
In May 2022, the FDA review of the EUA application resulted in rejection. The review concluded that the available clinical data do not provide sufficient clinical evidence of fluvoxamine efficacy in preventing progression to severe symptoms when given early during COVID infections. Additionally, neither the mechanism nor dosing regimen are clear.
CONCLUSION
While fluvoxamine might offer some benefit for early COVID infections, the need for its use is currently less urgent—especially after the availability of several antivirals. Further clinical trials are needed to determine the potential effectiveness of fluvoxamine as a repurposed inexpensive drug for early COVID treatment.