Zachary Pape, PharmD, BCACP; Abir T. El-Alfy, PhD, MS; Kristin Busse, PharmD, BCPS
WMJ. 2024;123:P1. Published online March 12, 2024.
On May 27, 2023, the US Food and Drug Administration (FDA) approved a new drug application, submitted by Lexicon Pharmaceuticals, Inc., for sotagliflozin (Inpefa), the first dual sodium-glucose cotransporter-1 and sodium-glucose cotransporter-2 inhibitor for the treatment of heart failure, including heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF).
INPEFA MECHANISM
Inpefa (sotagliflozin) acts as an inhibitor of both sodium-glucose cotransporter (SGLT) 1 and 2. Inhibition of SGLT1 reduces intestinal absorption of glucose and sodium, while SGLT2 inhibition results in reduced renal reabsorption of glucose and sodium. While the exact mechanism of the cardiovascular benefit is still unclear, decreasing renal sodium reabsorption can help reduce the cardiac preload/afterload and sympathetic activity.
FDA-APPROVED INDICATIONS
- Cardiovascular Risk Reduction: Including reduction of cardiovascular mortality, hospitalization for heart failure, and urgent heart failure visits in adults with type 2 diabetes, chronic kidney disease, and other cardiovascular risk factors.
- Heart Failure: Risk reduction of cardiovascular mortality, hospitalization for heart failure, and urgent heart failure visits in adults with heart failure.
DOSAGE AND ADMINSTRATION
Recommended Dosing:
- Initial: 200 mg by mouth once daily
- Maintenance: After ≥2 weeks, may increase to 400 mg by mouth once daily
ADMINISTRATION
- Tablets should not be taken more than 1 hour prior to first meal of the day.
- Tablets should not be cut, crushed, or chewed.
Cost (average wholesale price): 200 mg each: $23.92
ADVERSE EFFECTS
Trial results reported several potential adverse effects that may affect prescriber decisions on whether to utilize this newer agent. Adverse effects that either demonstrated high incidence (>10%) or statistically significant differences between sotagliflozin and placebo are:
- Diarrhea
- Urinary tract infections and genitourinary fungal infections
- Volume depletion
- Diabetic ketoacidosis
DRUG/FOOD INTERACTIONS
Sotagliflozin inhibits P-glycoprotein/ABCB, weakly induces CYP3A4, and is a minor substrate of CYP3A4.
- Sotagliflozin may increase serum concentration of afatinib, aliskiren, berotralstat, colchicine, cyclosporine, and digoxin through P-glycoprotein/ABCB1 inhibition. Dose adjustment and monitoring of these drugs are recommended when combined with sotagliflozin.
- Sotagliflozin may enhance the hypoglycemic effect of insulin and sulfonylureas.
- High-calorie meal increases sotagliflozin bioavailability and should be administered no more than 1 hour before the first meal of the day.
CLINICAL TRIALS
SOLOIST-WHF Trial (2021)
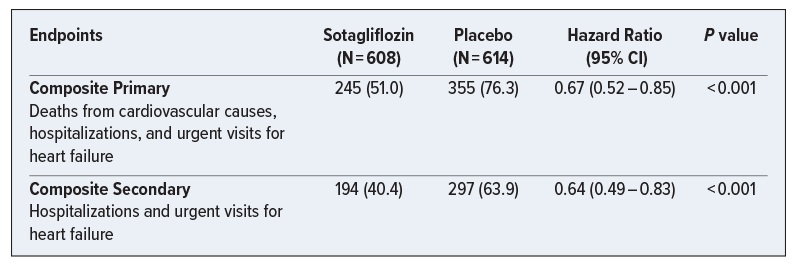
A Phase 3, multicenter, double-blind, randomized, placebo-controlled trial that included patients with type 2 diabetes mellitus who were recently hospitalized for worsening heart failure (as defined by the presence of signs and symptoms of heart failure and received treatment with intravenous diuretic therapy). Participants (N = 1222) were randomly assigned in a 1:1 ratio to receive sotagliflozin or placebo. While the trial was ended early due to funding issues, the findings below suggest that this new agent may benefit patients via reduction in combined cardiovascular (CV) outcomes (CV events and heart failure hospitalization) for patients with recent/worsening heart failure and type 2 diabetes when added prior to or shortly after discharge. Subgroup analyses suggest that sotagliflozin maintained its benefits across prespecified groups, with potentially greater confidence in groups such as those who were male, above the age of 65 years, or with estimated glomerular filtration rate < 60 ml/min/1.73m2.
SCORED Trial (2021)
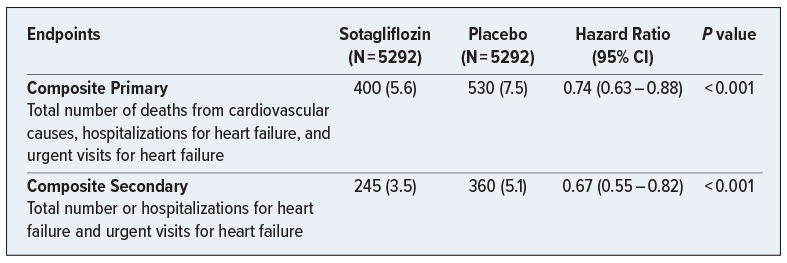
A Phase 3, multicenter, double-blind, randomized, placebo-controlled trial that included patients with type 2 diabetes and chronic kidney disease with risks for cardiovascular disease. The purpose of the trial was to evaluate the impact of sotagliflozin on CV outcomes for patients who met the study criteria. Participants (N = 10 584) were randomly assigned in a 1:1 ratio to receive sotagliflozin or placebo. This trial notably changed its composite endpoint criteria during the trial due to loss of funding from the sponsor, and the findings below suggest that this new agent may benefit patients via reduction in combined CV outcomes (CV mortality, heart failure hospitalizations, and urgent heart failure visits) and HF hospitalization individually for patients with type 2 diabetes and chronic kidney disease.
CONCLUSIONS
Sotagliflozin’s approval for heart failure and cardiovascular risk reduction appears to represent a significant development in the utility of SGLT-mechanistic agents specifically for the use of heart failure. Notably, sotagliflozin does not carry approved indications for either type 2 diabetes or chronic kidney disease alone versus other already available/approved agents (eg, canagliflozin, dapagliflozin, and empagliflozin). This new approval, and the evidence cited by the FDA, appear to cement its role in the management of heart failure of all types and has already been cited in the most recent American College of Cardiology (ACC) 2023 Expert Consensus Decision Pathway on the Management of HFpEF.